Haematological cancers: an integrated approach to detecting Acute Myeloid Leukaemia (AML)
The King’s Haematological Malignancy Diagnostic Centre (KHMDC) based at King’s College Hospital provides specialist diagnostic services for diagnosing haematological cancers. The department has an Immunophenotyping laboratory which processes approximately10,000 samples per year by flow cytometry, and a Cytogenetics laboratory which analyses samples by G-banded chromosome analysis and fluorescence in-situ hybridisation (FISH). Morphology, Histopathology and Molecular Genetics laboratories complete the department to assist diagnosis of haematological cancers.
Case Study of Patient A
This case study follows a sample taken from Patient A aged 61, through the different laboratories in the KHMDC. Patient A’s blood results showed that the white blood cell count (WBC) was within the normal range, a pancytopenia with haemoglobin, neutrophil and platelet count below normal levels. Pancytopenia can be caused by various diseases, including severe vitamin deficiencies, myelodysplasia, leukaemia, aplastic anaemia, viral infections and hypersplenism amongst others. Due to the clinical picture and an abnormal blood film, Patient A was suspected to have Acute Myeloid Leukaemia (AML) which can also be a cause of pancytopenia.
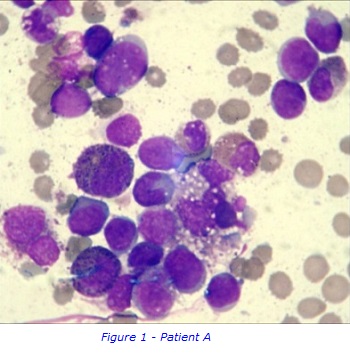
AML is a haematological malignancy where the bone marrow produces abnormal myeloblasts, an immature cell precursor. Because of a high number of leukemic cells in the bone marrow, normal blood cell production is replaced/suppressed resulting in , bone marrow failure. As a result, patients are often very anaemic, prone to infections and have a low platelet count.
Morphology
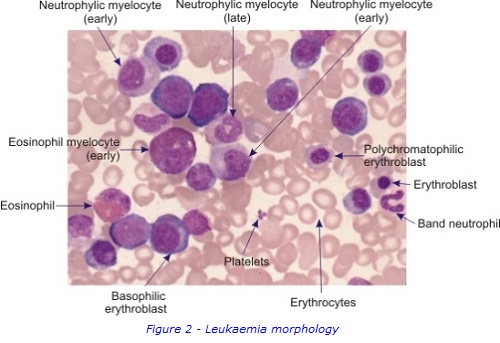
To collect bone marrow samples, a needle is inserted through skin and into the posterior iliac crest of the pelvic bone, under local anaesthetic. The liquid bone marrow aspirate withdrawn into a syringe and samples separated for testing in the different laboratories. . A solid sample (core) of bone marrow tissue is also taken with a larger trephine needle. A small sample of Patient A’s bone marrow aspirate was examined under a microscope to study the appearance of the cells and detect any abnormal morphology. It was confirmed that Patient A had increased numbers of early immature myeloblasts, which are shown in figures 1 & 2. This can be a sign of AML.
Immunophenotyping
Flow cytometry was used to examine Patient A’s sample and immunophenotyping showed an expanded population of CD34+ and CD34- myeloid progenitors along with an increase in promonocytes and eosinophils.
This abnormal excess of blasts and promonocytes in the bone marrow aspirate sample accounts for 25% of TNC’s. This is suggestive of AML as having at least 20% blasts in the marrow is consistent with acute leukaemia, with a FAB-subtype of AML-M4 with eosinophilia
Histopathology
Patient A’s bone marrow trephine was investigated in histopathology. The results from this solid biopsy showed an increase in the number of immature granulocytes. Due to this, erythropoiesis was markedly reduced to absent by morphology. Megakaryocytes were similarly reduced, but appear morphologically normal where present. Immunostaining with CD34, CD117 and CD123 was positive in 10-20%, ~20% and ~20% of TNC’s respectively with features consistent with AML.
Molecular Genetic Testing
The presence or absence of certain mutations can affect the prognosis in AML patients. Molecular genetic testing is therefore carried out in all patients with suspicion of AML. In the case of Patient A, polymerase chain reaction (PCR) was used to test for Nucleophosmin 1 (NPM1) insertion and FMS-like Kinase 3 internal tandem duplication (FLT3-ITD). Patient A’s molecular testing was negative for both the NPM1 and FLT3-ITD mutations, which is associated with an intermediate prognosis risk group.
Cytogenetic Testing
A G-banded karyotype was performed and showed an inversion of material in one chromosome 16 homologue. This is a recurrent finding in AML, resulting in fusion of the CBFB gene located at 16q22 to MYH11 at 16p13, and is consistent with the suspicion of AML-M4with increased eosinophils suggested by morphology. FISH studies confirmed this inversion of chromosome 16 using a fluorescent probe to visualise the gene fusion.
Conclusion
From the testing carried out by the various laboratories, Patient A was diagnosed as having AML with inv(16)(p13.1q22); CBFB-MYH11, which has a favourable prognosis.
Further information
Dr Robin Ireland
r [dot] ireland [at] nhs [dot] net

